Let’s talk!
The topic of birth control methods can be confusing. Even if you had sexual education classes in elementary or high school, the information may now be a vague memory. You are not alone, as most North Americans tend to have only a limited knowledge of the pill and condoms. One of the least discusses birth control methods is the intrauterine device (IUD) and the intrauterine system (IUS).
The first IUD (a device that fits entirely in the uterus) was marketed in 1929 by a German gynecologist named Ernst Gräfenberg. The ring-shaped IUD was made of silk threads, and only 3% of the 1,100 women who used it became pregnant. In 1930, he improved the device by covering the silk thread with a fine silver wire. Dr. Gräfenberg was unaware that the silver contained 26% copper, which reduced the pregnancy rate to 1.6% in 600 of the users. The copper provoked an inflammatory response in the uterus that created a hostile environment for sperm.
Over the years, various doctors improved the original IUD (see diagram below). For instance, the IUD became T-shaped, similar to the shape of the uterus, and this reconfiguration lowered its expulsion rate from the uterus. The device became smaller with one filament tail at its end, which made it more comfortable for women. By the 1970s, IUDs were made of plastic, with greater surface areas of copper, and consistently had pregnancy rates of less than 1%.
 IUDs are the world’s most widely used long-term reversible birth control method, with nearly 160 million users (about 2/3 of them being Chinese). In North America, only 2.9% of Canadian women and 1.8% of American women use IUDs. One of the reasons for its lack of popularity in the Western world is its perceived health risks.
IUDs are the world’s most widely used long-term reversible birth control method, with nearly 160 million users (about 2/3 of them being Chinese). In North America, only 2.9% of Canadian women and 1.8% of American women use IUDs. One of the reasons for its lack of popularity in the Western world is its perceived health risks.
In 1971, A.H. Robins Company manufactured and sold the Dalton Shield plastic IUD in North America. By 1974, this IUD was removed from the market by the US Food and Drug Administration (FDA) because there were reports of miscarriages, infections, infertility, and death resulting from using this device. The IUDs that are now available on the market do not put women’s health at risk. Fears of increased ectopic pregnancy or pelvic inflammatory disease are unfounded.
In North America, the two most popular types of IUDs are the copper ParaGard by Duramed Pharmaceuticals and the hormonal Mirena by Bayer.
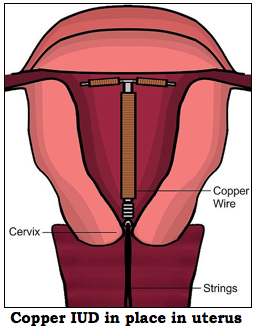
ParaGard T 380a is a T-shaped plastic frame wrapped with pure electrolytic copper wire and/or copper collars (see diagram above). The number “380†refers to the surface area in square millimeters of copper contained in the IUD. The top of the T is 32 mm wide and the vertical part is 36 mm long. The arms hold the device in place near the top of the uterus.
The exact manner in which the IUD prevents pregnancy is still unclear, but there are a few theories. For instance, the presence of ParaGard in the uterus provokes the release of leukocytes (white blood cells) and prostaglandins (involved in the inflammation response) by the uterus lining (endometrium). These substances are toxic to both sperm and egg while copper increases T 380a’s toxicity to sperm. Some doctors have suggested that ParaGard prevents the implanted embryos from developing in the uterus. It is, for this reason, an IUD can be used as emergency contraception if inserted up to 5 days after unprotected intercourse.
The T 380a can be left in the uterus for 10 years and costs about $250. In the long run, it is one of the cheapest birth control methods. The IUD needs to be inserted by a licensed physician. Many women complain of pain during the procedure and non-steroid anti-inflammatory drugs (NSAIDS) are recommended. Most women experience heavier and more painful periods after the insertion of an IUD. There are very rare complications such as the expulsion of the device by the uterus during the first year, and uterine perforation during insertion.
In the early 1970s, the first hormonal IUSs containing natural progesterone became available to women. New IUSs using synthetic progesterone were marketed in 1976. By the 1990s, IUSs were extensively used in Europe. In North America, it was not until the year 2000, the FDA approved the levonorgestrel-releasing intrauterine system (LNG-IUS) marketed under the brand name Mirena.
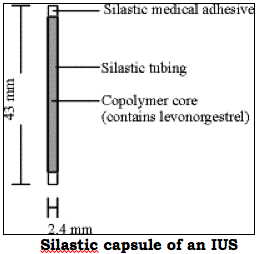
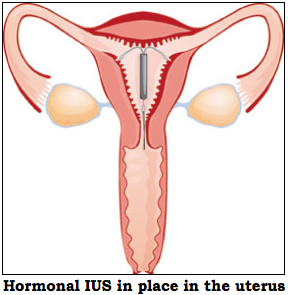 Mirena consists of an inert T-shaped polyethylene frame to which on the vertical part, is attached a polydimethylsiloxane reservoir (see diagrams below). The reservoir contains 52 mg of levonorgestrel. This synthetic progesterone is released gradually through a silastic capsule, at a rate of 20 µg/day. The daily amount of progesterone released by the capsule is much lower than the birth control pill (20 µg/day versus 0.1 mg/day).
Mirena consists of an inert T-shaped polyethylene frame to which on the vertical part, is attached a polydimethylsiloxane reservoir (see diagrams below). The reservoir contains 52 mg of levonorgestrel. This synthetic progesterone is released gradually through a silastic capsule, at a rate of 20 µg/day. The daily amount of progesterone released by the capsule is much lower than the birth control pill (20 µg/day versus 0.1 mg/day).
The LNG-IUS prevents pregnancy in a number of ways. In about 50% of women, the device stops ovulation. There is also a thickening of the cervical mucus in some users, which prevents the sperm from entering the uterus. Up to 66% of women have significant inflammation of the uterine wall, which may interfere with sperm function. Also, the endometrium produces Glycodelin A (a glycoprotein) in mid-cycle, which inhibits fertilization.
 A licensed physician needs to insert the Minera (ideally) during the first 7 days of the patient’s menstrual cycle. The IUS has an annual pregnancy rate of 0.1-0.2%, lower than the copper IUD and similar to sterilization. Other benefits are reduced menstrual flow, which is associated with an increase in hemoglobin and blood iron levels. The LNG-IUS costs about $250 and can stay in the uterus for up to 5 years, making it cost-effective.
A licensed physician needs to insert the Minera (ideally) during the first 7 days of the patient’s menstrual cycle. The IUS has an annual pregnancy rate of 0.1-0.2%, lower than the copper IUD and similar to sterilization. Other benefits are reduced menstrual flow, which is associated with an increase in hemoglobin and blood iron levels. The LNG-IUS costs about $250 and can stay in the uterus for up to 5 years, making it cost-effective.
IUDs have come a long way since the Gräfenberg ring. Pharmaceutical companies are testing new prototypes that will help reduce the negative side effects of the current devices. Women now have, more than ever, better control over their own fertility.
Literary Truths
You should not be using the IUD ParaGard T 380a if you have:
- A current pelvic infection
- A sensitivity to copper or nickel
- Concerns that you may be pregnant
- Have given birth within the past 4 weeks
- Benign or malignant gestational trophoblastic disease
- High likelihood of exposure to Gonorrhea and Chlamydia
- AIDS (unless under control with antiretroviral therapy)
- Endometrial, ovarian or cervical cancer
- Distortion of the uterus (i.e., fibroids)
- Unexplained vaginal bleeding
- A very recent septic abortion
You should not be using the LNG-IUS (Mirena) if you have:
- A current pelvic infection
- Concerns that you may be pregnant
- A weak immune system
- Liver disease
- Unexplained vaginal bleeding
- Uterine, cervical or breast cancer
- A history of breast cancer, ectopic pregnancy, tubal disease (i.e., pelvic inflammatory disease)
- A distortion of the uterus (i.e., fibroids)
- An allergy/sensitivity to levonorgestrel, silicone or polyethylene
Truth in Motion
References
Canadian Women Under-using IUDs
Contrel
Grigorieva V., M. Chen-Mok , M. Tarasova, et al. “Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas.†Fertil Steril, (2003);79:1194-1198.
Gynefix
The GyneFix Intrauterine Device
IUS diagram
“Long-acting progestogen contraceptives.” The Practitioner (2002): 332.
Paladine, Heather L., C. E. Blenning, and D. Zegar Judkins. “What are contraindications to IUDs?” Journal of Family Practice 55.8 (2006): 726.
Planned Parenthood of Toronto: IUD
Silastic Capsule diagram
Zinger, Michael, and M. A. Thomas. “Using the levonorgestrel IUS.†Contemporary OB/GYN 46.5 (2001): 35.
Thanks for sharing. Please keep updating this info. I like this site too much. Good website theme ;).
Thanks for the suggestion. 🙂
Regards,
J.M.
Hello. Remarkable job. I did not expect this. This is an impressive article. Thanks!
Beneficial info and excellent design you got here! I want to thank you for sharing your ideas and putting time into the stuff you publish! Great work!
Nice site, nice and easy on the eyes and great content too.
This information is really helpful to me and I’ll share it with a couple of friends. I will probably be checking back frequently to look for updates.
Amazing stuff. Thanx 🙂
I’ve been checking your blog for a while now, and it appears like everyday I learn something new. 🙂 Thanks
I believe that only few people possess the ability to write well and frankly, you have it. Genius!
I very much like reading your blog. Please keep posting such excellent content.
I really like this type of clever work! Keep up the excellent writing. I’ve added you to my blogroll.
Good article! Keep it up!
This website is cool; keep up the good work!
Thank you ever so for you blog post. Great!
Abundance of fun, that’s how I call sites like this ;). Cheers for the great stuff you published, I’ll see some more and I hope it’ll have comparable quality ;).
I was searching for this info for a while. After 6 hours of continuous Googleing, at last I found it on your website. I wonder why Google didn’t rank this kind of informative sites at the top of the search list. Generally the top sites are full of garbage.
I like the valuable information you provide in your articles. I will bookmark your weblog and check again here frequently. I am quite certain I will learn lots of new stuff right here!
Awesome article once again! I am looking forward to more updates:)
Hello there! I really enjoy reading your blog! If you keep making amazing posts like this I will come back every day to keep reading.
Great post and very informative. I wonder why the other specialists of this sector do not notice this. You must continue your writing. I’m confident you already have a great readers’ base!
Thanks so much for the article. Really Cool.
A big thank you for your blog.